23.12 Definition of the word “diamond.” (U.S. FTC, Federal Trade Commission)
DIAMONDS DEFINITION
What is a diamond What is the definition of diamonds? What are diamonds made of? What material are diamonds? Must be real diamonds from the mine? What are natural diamonds, natural diamonds? Are Lab Grown Diamonds, lab diamonds real diamonds? Are Lab Grown Diamonds, lab diamonds identical to mine diamonds? Are there any law articles on Lab Grown Diamonds? Is there any evidence that Lab Grown Diamonds are really real diamonds?
(a) A diamond is a mineral consisting essentially of pure carbon crystallized in the isometric system. It is found in many colors. Its hardness is 10; its specific gravity is approximately 3.52; and it has a refractive index of 2.42.
Laboratory-created stones (also called laboratory-grown, or manufacturer-created stones) have the same chemical, physical, and visual properties as natural gemstones, but they are manufactured.
Summary of Basis and Purpose for the Revised Jewelry Guides – August 8, 2018
Eliminate the word “natural” from the diamond definition
The final Guides therefore eliminate the word “natural” from the diamond definition. When the Commission first used this definition in 1956,432 there was only one type of diamond product on the market: natural stones mined from the earth. Since then, technological advances have made it possible to create diamonds in a laboratory. These stones have essentially the same optical, physical, and chemical properties as mined diamonds. Thus, they are diamonds.
The distinctions between these lab-created diamonds and mined stones are addressed elsewhere in the Guides.433 Because it is no longer correct to define diamonds as “natural,” the final Guides do not include “natural” in the diamond definition.
What is a diamond
DIAMOND MATERIAL
What is a diamond What material are diamonds made of? What material are Lab Grown diamonds made of? What are Lab Grown Diamonds made of? What is the chemical composition of a diamond? Did Lab Grown Diamonds differ chemically from diamonds from the mine?



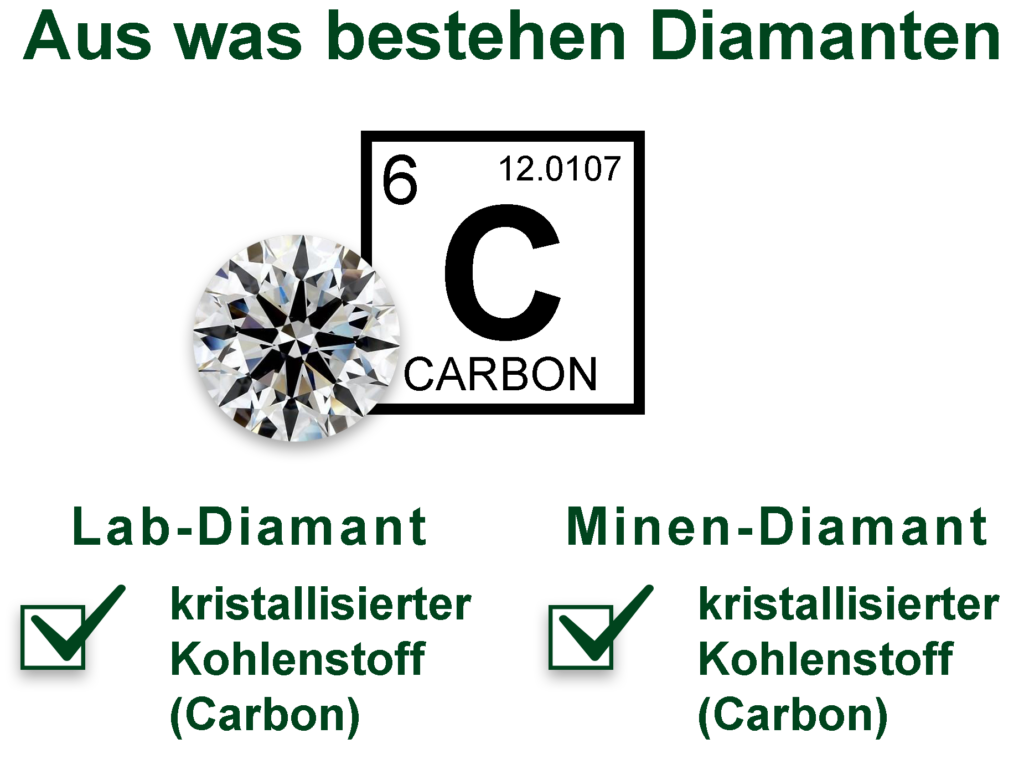
All genuine diamonds consist of:
Crystallized carbon
(Pure Carbon, cubic C)
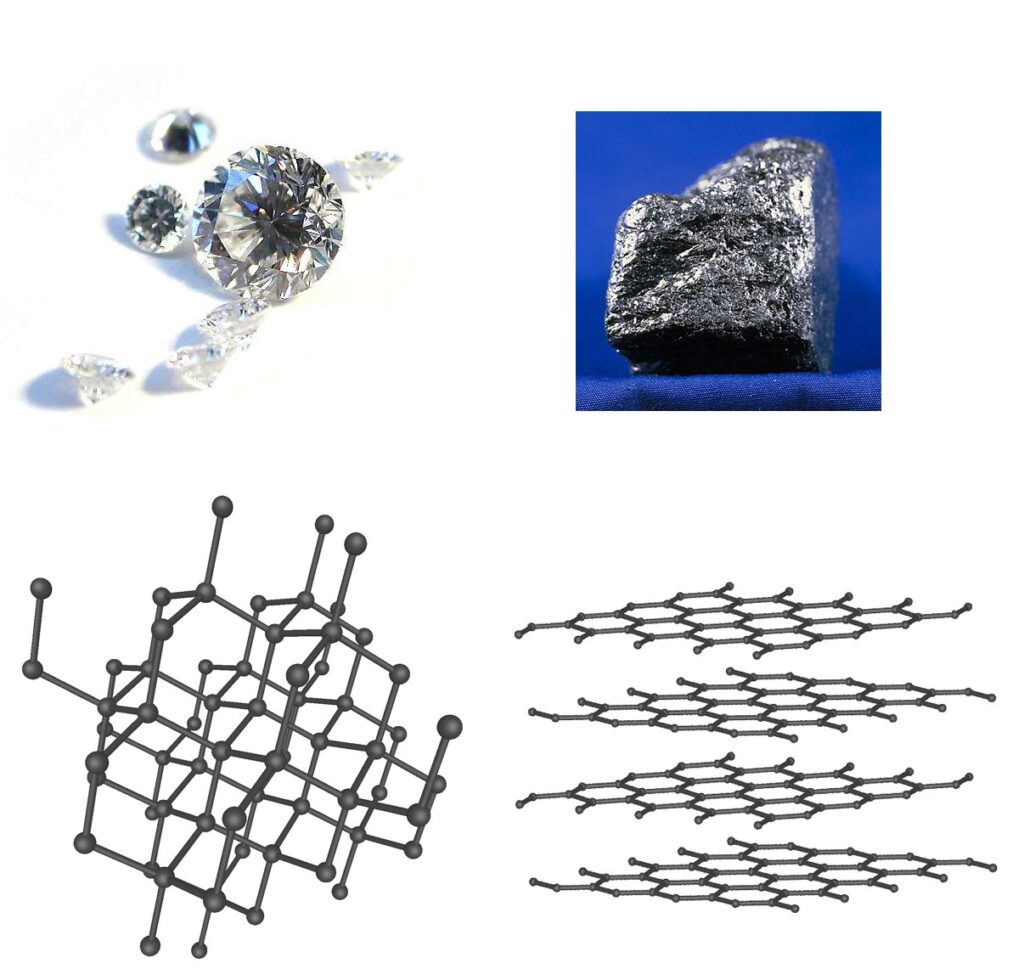
Every real diamond consists of pure crystallized carbon, the atomic lattice structure is cubic, i.e. the atoms are arranged in a cube shape at right angles (Pure Carbon, cubic C).
Diamond is the cubic modification of carbon and, as a naturally occurring solid, is a mineral from the mineral class of the elements. Diamond usually forms octahedral crystals, often with curved and streaky surfaces. Other shapes observed are the tetrahedron, dodecahedron and the cube. The crystals are transparent, colorless or colored green, yellow, brown and, more rarely, orange, blue, pink, red or gray to black due to impurities (e.g. nitrogen or boron) or crystal lattice defects.


Carbon (chemical element “C”)
Essential element of the biosphere
Carbon (Latinized carboneum or carbonium) is a natural substance that occurs frequently on earth. Carbon is a chemical element with the element symbol C and the atomic number 6. It occurs in nature both in a dignified (pure) form (diamond, graphite, chaoite) and chemically bound (e.g. in the form of carbides, carbonates, carbon dioxide, petroleum, Natural gas and coal). Carbon is an essential element of the biosphere; in all living beings – after oxygen (water) – it is the most important element in terms of weight. All living tissue is made up of (organic) carbon compounds. Geologically, on the other hand, carbon is not one of the most common elements, because the mass fraction of carbon in the earth’s crust is only 0.027%.
Diamond is the hardest natural substance (10 Mohs scale)
High light refraction, high dispersion, hardest substance on earth -> practically indestructible
Diamond is the hardest natural substance
In the Mohs hardness scale, it has a hardness of 10
What is the hardest material on earth? Are diamonds the hardest material on earth? Are Lab Grown Diamonds the same hardness as mine diamonds? Are diamonds indestructible? Do Lab Grown diamonds have the same longevity as mine diamonds?
Its grinding hardness according to Rosiwal (also absolute hardness) is 140 times greater than that of corundum. However, the hardness of the diamond is different in different crystal directions (anisotropy). This makes it possible to cut diamond with diamonds. In the diamond powder used for this purpose, the crystals are in every orientation (statistical isotropy), so that the hardest of them always act on the body to be ground.
Diamond is optically isotropic with high light refraction and high dispersion
It sometimes shows fluorescence and phosphorescence and is triboelectric. It has the highest thermal conductivity of all known minerals.
The weight of individual diamonds is traditionally given in carats, a unit that corresponds exactly to 0.2 grams (see section “Weight in carats”). An untreated diamond, ie in particular an uncut diamond, is called a rough diamond.
Hardest substance on earth
practically indestructible
Can you break a diamond? Can you destroy a diamond?
The diamond is the hardest natural substance on earth. On the Mohs hardness scale, it has a hardness of 10. It can cut any kind of rock or metal, but only another diamond can cut a diamond . To burn a diamond, it has to be heated to between 1,290 and 1,650 degrees Celsius. But the oil that is deposited by the mere touch of a human finger can cause dirt to build up and this almost indestructible gemstone quickly loses its sparkling charm.
DIAMONDS CHEMICAL PROPERTIES
Do Lab Grown Diamonds have the same properties, chemical composition, structure, refractive index, dispersion, hardness, density as mine diamonds?
| Diamond Property | Mined Diamond | Lab Grown Diamond |
|---|---|---|
| Chemical Composition | C (Crystallized Carbon) | C (Crystallized Carbon) |
| Crystalline Structure | Cubic | Cubic |
| Refractive Index | 2.42 | 2.42 |
| Dispersion | 0.044 | 0.044 |
| Hardness | 10 | 10 |
| Density | 3.52 | 3.52 |
Diamond versus gold
Difference diamond versus gold (periodic table of chemistry)
What is the difference of diamonds and gold? Why can you make diamonds in the lab and not gold?
The short answer is: diamonds are not the chemical element itself, but are a special form (crystallized, cubic) of the chemical element “C” carbon. Gold is the chemical element “AU” itself. This essential difference makes it possible to grow Lab Grown Diamonds.
“C” Carbon
Many people think a diamond is a commodity like gold.
-> That’s not entirely correct.
Diamond = crystallized carbon -> chemical element = “C” Carbon
Diamond
= crystallized from of carbon / graphite
= chemically most stable (atoms arranged cubically / wavelike (right angles)) and most expensive form
Diamond = crystallized carbon -> chemical element = “C” Carbon (not the diamond itself).
Gold = chemical element “AU”.
The material of diamonds is crystallized carbon! That is, the most valuable form of carbon.
“Carbon” (C) is the element of the chemical periods table.
A diamond in this form is not an element of the chemical periods table like gold (AU).
TECHNOLOGICAL ADVANCEMENT
Technology brings with it innovations, inventions and advances from which we can benefit.
Technical possibility for the production of diamonds
Many people think diamonds are like gold -> both a commodity, both from the ground.
Fortunately, there is a crucial difference to which we owe the fact that the production of diamonds is technically possible, but that of gold is not (yet?).
The diamond is a special form (= cubish) of carbon and not the chemical element itself from the chemistry periods table (= carbon) fundamentally distinguishes the diamond from gold (= chemical element AU).
This fact opens the technical possibility for the production of diamonds.
That is, if you technically manage to convert the chemical element carbon (C) into its most valuable form “crystallized carbon”, then you have created a diamond.
For example, carbon in the form of “graphite (= pencil point) “with soft plate-like arranged atomic structure -> in “diamond” with cubic cube-shaped very stable arranged atomic structure.
Conversion of carbon (C) -> into “crystallized carbon (cubic C)” = diamond.
Cool?!
Gold = chemical element “AU” of the periodic table of chemistry
With gold (AU), on the other hand, you would have to be able to split atoms to make it, and so far that has not been possible.
What is gold? What is gold made of? Gold is the element “AU” (Latin aurum) of the periodic table of chemistry.
With gold (AU), on the other hand, you would have to be able to split atoms to make it, and so far that has not been possible. Early in history, alchemists had tried this, but failed.
Lab-Grown Diamonds = same material, same atomic lattice structure
Are Lab Grown Diamonds made of the same material as diamonds from the mine?
Are there differences between Lab Grown Diamonds and mine diamonds?
Can you see differences from Lab Grown Diamonds to those from the mine?
Lab grown diamonds are made of the same material (= crystallized carbon) as mine diamonds and have the same atomic lattice structure. This means that the carbon atoms are arranged in the same way in both rennet diamonds and mine diamonds = cubic (cube-shaped)!
What more could you want?
Same material, same atomic lattice structure – optically, chemically and physically identical!
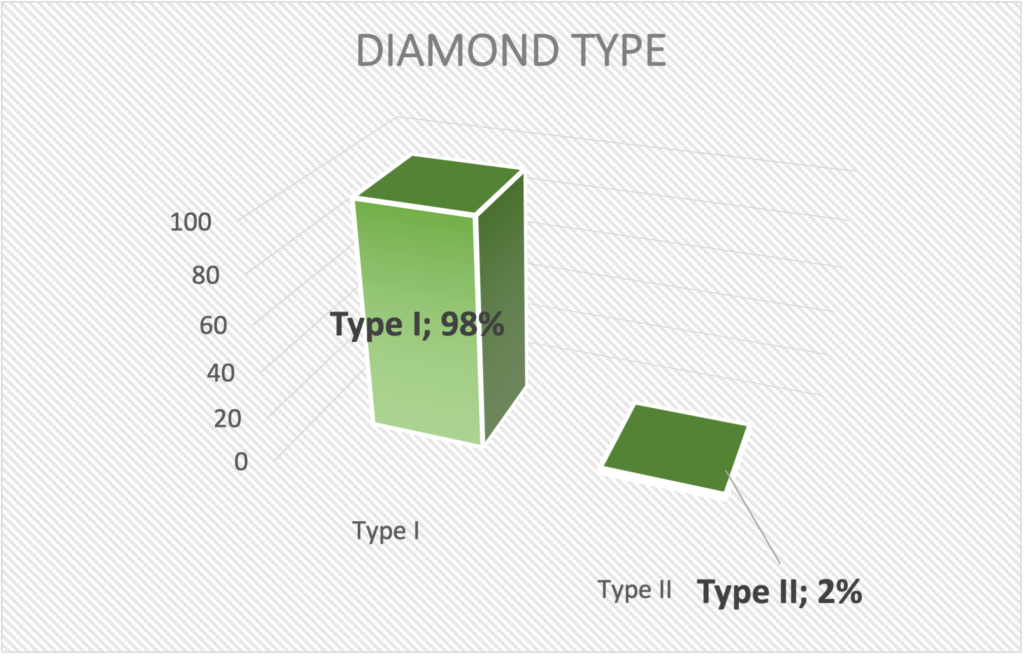
TYPE I and TYPE II DIAMONDS
Are there different diamond types? What are the types of diamonds? What is a Type I Diamond? What is a Type II diamond? What are Type IIa diamonds? Is a Type II diamond better than a Type I? Are Type II diamonds rarer than Type I? What is a Type IIa Lab Grown Diamond?
98% of all diamonds are Type I diamonds which contain nitrogen (nitrogen). In yellow diamonds, the nitrogen gives the beautiful yellow color. In white diamonds, nitrogen is an impurity, so in white diamonds, Type I diamonds are inferior and less valuable than Type II diamonds.
Type IIa diamonds are the most valuable and chemically pure type of diamonds with exceptional optical transparency. They are almost 100% nitrogen-free. Only 2% of all mine diamonds are Type IIa, so it is a very rare, valuable and exclusive group of diamonds.
Type II diamonds are purer, rarer, more desirable and more expensive. Type II diamonds are virtually nitrogen free. There are white, brown, pink / pink, red type IIa diamonds and blue type IIb diamonds.
Type II (only 2% of mine diamonds = purest, most expensive and most sought after white diamonds).
Virtually all rennet diamonds are Type II. ….Thus, they are among the best 2% in terms of quality, with virtually no nitrogen impurities.
Diamonds are mainly made of carbon. However, there are other elements that are part of the diamond’s internal structure, and these affect the color grade of fancy color diamonds.
- Type I
- Diamonds WITH nitrogen (N) atoms (= “nitrogen group”)
- approx. 98% of all mined diamonds
- IaAB, IaA, IaB and Ib (depending on the way nitrogen combines with carbon in the internal structure of the diamond).
- white diamonds → nitrogen in them is not “light-absorbing
- e.g. yellow (Yellow), orange (Orange) → nitrogen in them absorbs color
- brown, in some blue & green & in australian pink diamonds
- Type II
- almost 100% nitrogen-free (= WITHOUT nitrogen)
- approx. 2% of all mine diamonds → very rare group of diamonds
- Type IIa
- purest diamonds = consist almost entirely of carbon
- they can be colorless (White), brown (Brown) or pink (Pink)
- Type IIb
- ultra-red and nitrogen-free
- blue fancy diamonds (Fancy Color BLUE)
- contain boron (B) atoms → this boron in the otherwise pure carbon atom structure is responsible for the blue color in blue fancy diamonds
- conduct electricity
Difference diamond versus zirconia and moissanite
What are diamond simulants? What are diamond imitations?
What is a zirconia? Is a cubic zirconia a real diamond or a fake diamond? Is a cubic zirconia the same as a Lab Grown diamond?
What is a Moissanite? Is a moissanite a real diamond or a fake diamond? Is a Moissanite the same as a Lab Grown diamond?
What is the difference between Lab Grown Diamonds and synthetic diamonds like Zirconia and Moissanite?
Are Lab Grown Stones the same as Lab Grown Diamonds?
Attention!
Unfortunately, there are suppliers who work with fake Lab Grown Diamonds and try to deceive the consumer. Lab Grown Stone does not necessarily mean Lab Grown Diamond. We have seen plenty of other descriptions that have nothing to do with real diamonds and nothing to do with Lab Grown Diamonds.
Synthetic Cubic Zirconia (CZ)
No, Zirconia are not the same as Lab Grown Diamonds. Zirconia are not real diamonds!
Zirconia are made of zirconium(IV) oxide. They are very cheap fake diamonds “Diamond Simulants”. Repeated contact with water will damage the stone. This is a completely different material and never has the hardness and longevity of a diamond (8 versus 10). Cubic zircon will last two to three years with daily wear, as long as you clean and care for your jewelry. With occasional wear, a cubic zirconia can last up to five years. Over time, zirconia usually becomes scratched and cloudy. Cubic zirconia becomes cloudy over time due to scratches, soap and mineral residues, dirt, and exposure to oxygen in air and water.
Synthetic Moissanite (SIC)
No, Moissanite is not the same as Lab Grown Diamonds. Moissanites are not real diamonds!
For laymen, moissanites are more difficult to distinguish from real diamonds.
Moissanites are made of silicon carbide. These are also not diamonds, but an imitation “Diamond Simulants”. This is also a very different material and although very hard, does not have the hardness (9.25 versus 10) and properties of diamonds. Moissanite is closest in physical properties to diamond than any other diamond simulant. But are clearly fake diamonds and can be distinguished by experts.
| Property | Synthetic Cubic Zirconia | Synthetic Moissanite | Mined Diamond & Lab Grown Diamond |
|---|---|---|---|
| Chemical Composition | Zirconium(IV) oxide | Silicon Carbide | C (Crystallized Carbon) |
| Crystalline Structure | cubic crystalline form of zirconium dioxide (ZrO2) | Hexagonal | Cubic |
| Refractive Index | 2.15 | 2.65-2.70 (double refractive -> “doubling”/fatamorgana effect) | 2.42 |
| Dispersion | 0.060 | 0.104 (very strong) | 0.044 |
| Hardness | 8 | 9.25 | 10 |
| Density | 5.95 | 3.22 (floats on methylene iodide, diamond sinks) | 3.52 |
